A UK–China Perspective on Responsible Innovation
Prepared by Excellence First Enterprise Consultancy (EFEC)
Introduction:
Why ESG Matters in Cross-Border Life Sciences
In life sciences, innovation is not only about scientific discovery or commercial success—it
directly impacts patients, ecosystems, and public trust. In this context, Environmental, Social,
and Governance (ESG) principles are now considered foundations of credible and sustainable
collaboration.
For UK and China, two of the world’s most dynamic and complementary innovation ecosystems,
ESG is not an optional extra. Cross-border partnerships face heightened scrutiny, from differing
regulatory systems to cultural expectations. ESG provides the framework that allows collaborations
to move beyond transactions and become long-term, trusted relationships.Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
What ESG Means in Life Sciences
Environmental
Clinical trial sustainability:
reducing travel footprints, minimising waste, and adopting greener
logistics.
Greener labs:
adopting low-energy equipment, sustainable sourcing, and environmentally friendly practices
Transparent supply chains:
ensuring vendors and partners align with international green standards.
Social
Fairness in partnerships:
equitable terms for both SMEs and larger institutions
Patient-centred outcomes:
prioritising patient safety, accessibility, and public health relevance
Workforce inclusion:
ensuring diverse, inclusive teams across research, clinical, and commercial levels.
Governance – Ethics, Transparency and Trust
Data integrity:
applying rigorous stewardship, transparency, and accountability in cross-border trials
Intellectual property protection:
Securing and respecting IP rights in both UK and China.
AI/Biotech ethics:
embedding algorithmic transparency, informed consent, and ethical compliance in
advanced technologies.
Practical Steps for SMEs & Founders
For SMEs and startups, ESG can feel abstract, but embedding it early builds credibility with
partners and investors. Practical steps include:Clinical trial sustainability:
reducing travel footprints, minimising waste, and adopting greener
logistics.
- Embed ESG metrics in pitch decks and MoUs.
- Select CROs, service providers, and vendors with documented ESG commitments.
- Apply ‘data ethics by design’ in trials, especially AI-driven studies.
- Monitor diversity and inclusion across teams and partnerships.
- Report transparently on sustainability and patient outcomes.
- Build scalable ESG frameworks, including self-audits, to demonstrate progress.
EFEC’s Role in Embedding ESG
EFEC maintains a dynamic network of independent, accredited ESG, legal, and regulatory
experts in both the UK and China. All guidance is validated in direct collaboration with these
specialists to ensure clients receive the most up-to-date, credible, and cost-effective advice and
solutions.
Excellence First Enterprise Consultancy Ltd (EFEC) is not just an advisory body, but a valuesdriven catalyst for responsible growth in life sciences. Our contributions include:
Partnership Design:
Ensuring ESG is integrated into alliance structures and agreements.
Talent Pipelines (CognateUK)
Building a new generation of globally minded leaders trained in ESG principles.
Innovation Hubs (UK–China Life Sciences Innovation Hub)
Embedding ESG awareness in ecosystem projects from inception.
EFEC’s mission is to ensure that cross-border ambition is matched with responsibility, fairness,
and trust.
For founders and ecosystem leaders, ESG is now a non-negotiable differentiator.
Embedding ESG not only improves compliance and credibility, it makes
partnerships more resilient and impactful.
EFEC invites innovators, investors, and institutions to co-create ESG-centred pathways across
the UK and China. Together, we can ensure that science serves patients and society while
building sustainable, cross-border ecosystems.
Our Three Pillars
Environmental – Supporting Sustainable Innovation
The Hub works with organisations that recognise sustainability as a foundation for innovation. Across our partnerships, we promote environmentally conscious operations, responsible resource use, and sustainable modes of collaboration. We encourage our partners to consider the full lifecycle of innovation — from research and clinical validation to data infrastructure and market access.
Our role is to amplify what’s already working — helping ecosystems align sustainable growth with real-world impact.
Social – Building Trust and Capability Across Cultures
Our social responsibility begins with people. We help organisations and innovators operate confidently across borders by embedding cultural fluency, inclusion, and shared understanding into every collaboration.
Through our in2UK and in2China services, we support businesses entering each other’s markets with the right context, connections, and confidence. We focus on bridging not just opportunity gaps, but also differences in expectations, communication, and professional practice.
We prioritise fairness, opportunity, and mutual respect in all our partnerships — creating environments where intelligence, talent, and purpose can thrive.
Governance – Ethics, Transparency and Trust
Trust is the currency of cross-border innovation. The Hub ensures that partnerships between UK and Chinese institutions are guided by clear governance principles, transparent processes, and respect for data, regulation, and intellectual property.
We align our approach with global best practice in compliance and ethics, while remaining sensitive to local contexts. Our governance philosophy is simple: collaboration should strengthen integrity, not test it.
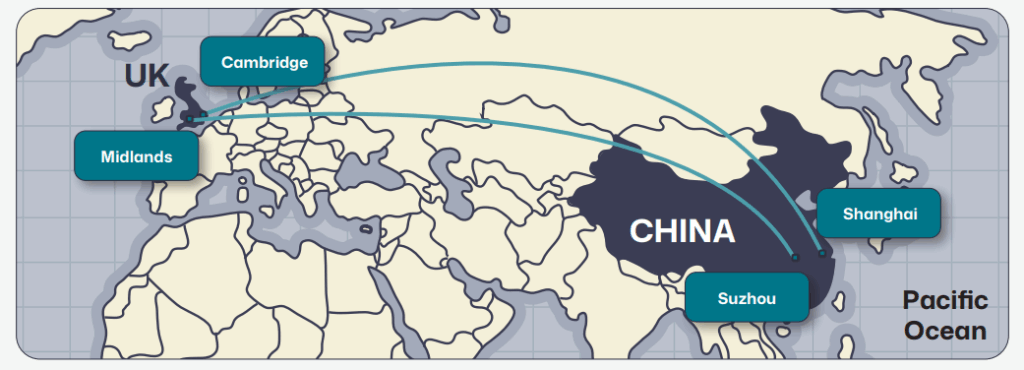
UK–China map with arrows showing collaborative ESG touchpoints (e.g., Cambridge ↔ Shanghai, Midlands ↔ Suzhou).
Founder-Facing Checklist
For SMEs and startups, ESG can feel abstract, but embedding it early builds credibility with
partners and investors. Practical steps include:Clinical trial sustainability:
reducing travel footprints, minimising waste, and adopting greener
logistics.
- Engage NMPA and provincial pilot authorities early.
- Select the right pilot zone for your product (Hainan = imports, Lingang = AI/real-world, Suzhou = biotech collabs).
- Partner with local CROs — bilingual, regulatory-savvy, trusted
- Translate and localise all trial documentation
- Budget realistically: CRO fees, site audits, translations, legal/IP.
- Secure IP in both UK and China before entry.
- Plan MHRA and NMPA pathways in parallel.
- Prioritise early regulator engagement and transparency.
- Involve EFEC as a bridge and interpreter — regulators respond better when intermediaries are trusted.






